ISO 9001:2015
Quality Management System
The Quality Management System that grow the organization | ISO 9001 Certification in 7 Working Days

Success through management excellence

The Power of ISO 9001 Certification
In today’s competitive business landscape, not only must organizations prioritize efficiency, consistency, and customer satisfaction, but also they need a proven system to sustain these standards. Fortunately, ISO 9001 Certification or ISO 9001 Registration or quality certification serves as the gold standard for quality management systems (QMS), since it helps businesses streamline operations, minimize risks, and enhance credibility. However, achieving certification not only requires expertise but also demands structured planning and hands-on implementation support.
This is precisely where Global Standards excels. As a result, as a trusted certification service provider, we guide organizations through every step of the ISO 9001 Certification process—starting from initial assessments all the way to final accreditation. Moreover, our proven methodologies have delivered 100% success rates across industries, including healthcare, pharmaceuticals, and logistics.
Therefore, this white paper explores exactly how Global Standards helps businesses achieve ISO 9001 Certification efficiently. For instance, we showcase real-world success stories from Dr. Ziauddin Hospital, Otsuka Pakistan Ltd, and Pak Shaheen Container Services, thereby demonstrating measurable improvements in quality, compliance, and operational performance.
Why ISO 9001 Certification is a Game-Changer
The Transformative Benefits of ISO 9001 Implementation
Organizations implementing ISO 9001 gain numerous advantages, specifically including:
- First and foremost, enhanced customer trust – since certification demonstrates reliability, thereby increasing client retention.
- Additionally, operational efficiency improves because standardized processes reduce waste while simultaneously minimizing errors.
- Furthermore, regulatory compliance becomes stronger as meeting international standards helps avoid legal risks.
- Most importantly, companies gain a competitive edge when certification makes them stand out in tenders and consequently in global markets.
However, despite these clear benefits, many businesses face implementation challenges primarily due to:
- Unclear guidelines
- Insufficient employee training
- Ineffective auditing processes
This is exactly where Global Standards makes the difference. By implementing a structured, sector-specific approach, we successfully eliminate these common hurdles.
Our Proven Methodology for ISO 9001 Certification Success
To ensure seamless certification with minimal operational disruption, we follow this comprehensive process:
1. Initial Gap Analysis
Before beginning, we conduct thorough assessments in order to identify compliance gaps. This crucial step establishes the foundation for customized action plans.
2. Customized Training Programs
Rather than offering generic training, we instead provide industry-specific workshops. This approach guarantees employees fully understand ISO 9001 requirements within their unique operational context.
3. Documentation & Process Optimization
Next, we assist businesses in developing clear SOPs that not only align with ISO 9001 standards but also eliminate workflow ambiguities.
4. Internal Audits & Continuous Improvement
Prior to final certification audits, we proactively conduct mock assessments. These evaluations help refine processes while addressing potential weaknesses.
5. Certification & Post-Certification Support
Unlike other providers, we maintain client relationships post-certification. Through ongoing support, we ensure continuous compliance and sustained improvement.
Unlock Business Excellence with ISO 9001 Certification in Pakistan
Why Your Business Needs ISO 9001 Certification in Pakistan
Ultimately, every forward-thinking company in Pakistan seeks a powerful competitive edge. Consequently, achieving ISO 9001 certification in Pakistan is the definitive strategy for superior quality management. This framework fundamentally enhances your operational processes; moreover, it systematically builds incredible customer trust and loyalty. Therefore, pursuing ISO 9001 certification in Pakistan directly translates to improved market reputation and streamlined efficiencies.
The Tangible Benefits of Your ISO 9001 Certification
Furthermore, the advantages of securing your ISO 9001 certification in Pakistan are both immediate and profound. First, it consistently opens doors to new markets and global supply chains. Then, it dramatically reduces costs by eliminating waste and preventing errors. Additionally, a valid ISO 9001 certification in Pakistan strengthens your tender applications and gives you a decisive advantage over competitors.
Your Guaranteed Path to Success with Global Standards
However, navigating the certification process requires expert precision. This is precisely why Global Standards operates as your dedicated certification partner. We expertly demystify every requirement for our clients. Our consultants provide a clear, step-by-step roadmap; furthermore, they prepare your entire organization for the audit. As a result, our proven methodology ensures a seamless journey and a 100% success rate for your ISO 9001 certification in Pakistan.
Take the Decisive Step Toward Growth Today
Ultimately, we transform this complex achievement into a straightforward business advantage. So, stop considering the benefits and start realizing them. Partner with Global Standards today and secure your ISO 9001 certification in Pakistan to unlock unparalleled growth, quality recognition, and sustainable success.
ISO regularly reviews all management system standards to ensure market relevance. Following an extensive user survey, the technical committee identified key improvement areas and consequently established long-term objectives. Importantly, these updates maintain stability for ten years, allowing organizations to fully implement durable quality management systems. Therefore, businesses can confidently adopt ISO 9001 certification, knowing the framework won’t change prematurely. Ultimately, this approach balances innovation with consistency, empowering companies to build robust QMS foundations.
Elevate Your Regional Standing with ISO 9001 Certification in the Middle East
Ultimately, the Middle Eastern market demands unparalleled quality and reliability. Consequently, achieving ISO 9001 Certification in the Middle East is a critical step for any business aiming for regional leadership. This internationally recognized standard fundamentally transforms your quality management system. Moreover, it systematically enhances customer satisfaction and operational consistency across diverse sectors. Therefore, pursuing ISO 9001 Certification in the Middle East directly strengthens your competitive position and institutionalizes excellence.
Secure a Tangible Return on Investment from Your Certification
Furthermore, the strategic benefits of securing your ISO 9001 Certification in the Middle East are profound. First, it builds formidable trust with government entities and multinational partners. Then, it consistently unlocks lucrative tender opportunities that require certified quality systems. Additionally, a valid ISO 9001 Certification in the Middle East streamlines processes to reduce costs and drive profitability, making your organization leaner and more resilient.
Guarantee Your Success with Our Regional Expertise
However, navigating the local compliance landscape requires a specialized partner. This is precisely why Global Standards is your ideal partner for ISO 9001 Certification in the Middle East. We possess deep, regional expertise and expertly guide you through every step. Our consultants handle all documentation and preparation with precision; furthermore, we ensure your team is fully prepared for the audit. As a result, our tailored methodology guarantees a seamless journey and a 100% success rate for your ISO 9001 Certification in the Middle East.
Command the Market with Your Certified Quality System
Ultimately, we transform this rigorous process into a direct strategic advantage. So, stop planning for quality and start demonstrating it. Partner with Global Standards today and secure your ISO 9001 Certification in the Middle East to command higher trust, win more business, and achieve sustainable market dominance.
Success Stories: How Global Standards Delivers Results
There are number of sectors to whom we have delivered our 100% services i. e. Engineering, NGO, Laboratories, Financial institutes, Textiles, I.T, Hospitals and so many in which for the understanding example of three clients has been mentioned for the reference.
1. Hospital Sector – Dr. Ziauddin Hospital
Challenge:
The hospital needed to strengthen its quality assurance systems and staff competency to meet international healthcare standards.
Solution:
- Accredited Training: We conducted specialized workshops for medical and administrative staff.
- QMS Restructuring: We redesigned workflows to align with ISO 9001 requirements.
- Leadership Engagement: Hospital management participated in strategy sessions to drive compliance.
Results:
- 100% ISO 9001 Certification or quality certification within six months.
- Reduced patient complaints due to standardized care procedures.
- Higher employee efficiency with clear role definitions.
“ Worked with global …wonderful company and great input, resulted in positive outcomes. TRUSTWORTHY AND DEDICATED PEOPLE.” – Dr. Ziauddin Hospital Administration
2. Pharmaceutical Sector – Otsuka Pakistan Ltd
Challenge:
The company needed rapid QMS improvements to meet strict pharmaceutical regulations.
Solution:
- Leadership Training: We coached executives on driving a quality-centric culture.
- Process Optimization: We eliminated redundant steps in production and documentation.
- Risk Management: We implemented proactive error-prevention strategies.
Results:
- 70% improvement in QMS within six months.
- Faster regulatory approvals due to compliant documentation.
- Higher supplier confidence in product quality.
“ Working with Global Standards on our upgrade to ISO 9001, ISO 14001 and ISO 45001 was a great experience. The Concentric tool simplified the transition and replaced complex documents with clear visual tools, making compliance and improvement much easier.” – Otsuka Pakistan Ltd Management
3. Off-Dock Terminal Sector – Pak Shaheen Container Services
Challenge:
The terminal’s initial QMS audit revealed only 40% compliance, leading to operational inefficiencies.
Solution:
- Employee Training: We educated staff on ISO 9001 requirements for logistics.
- Process Documentation: We created clear guidelines for cargo handling and tracking.
- Internal Audits: We identified and resolved bottlenecks before the final audit.
Results:
- 80% improvement in QMS within a year.
- Fewer shipment errors due to standardized procedures.
- Increased client trust in service reliability.
“Thanks to Global Standards, we now operate at an internationally recognized quality level.” – Pak Shaheen Container Services Leadership
© Global Standards. All rights reserverd for this documented information shared for reading purpose only.
White Paper - ISO 9001:2015 Quality Management System
BENEFITS OF ISO 9001 CERTIFICATION
Just in 7 Working Days
Achieve Consistency and Compliance
Achieve consistency of product and service quality and compliance with legal and associated requirements of interested parties.
Formalize Good Working Practices
Formalize good working practices through better planning.
Assure Satisfaction and Added Value
Assure satisfaction and added value to interested parties and features of existing services.
Introduce Risk-Based Thinking
Introducing a Risk-based thinking approach as preventive action and to promote a Proactive Approach.
Understand and Monitor Needs
Understanding and monitoring needs and expectations of interested parties.
Gain International Recognition
Be internationally recognized as a well-managed organization and business holder for quality management system.
Increase Promotion of Products and Services
To increase the promotion of products and services through this standard’s tools.
Ensure Employee Knowledge and Execution
Employee knows what to do and how to execute.
Improve Management Controls
Better management controls through the quality team.
Monitor Quality-Assured Environment
Monitoring of a quality-assured working environment.
Enhance Credibility
Increase credibility among business associates.
Build Confidence
Be confident through internal auditing, organizational knowledge, and management reviews.
Encourage Continual Improvement
Achievement of goals through encouraging continual improvement.
The QMS 9001 is based on the management Principles as shown below.
1 – Focus on customer and interested parties
2 – Provide leadership for your organization
3 – Engage and involve your people
4 – Use a process approach
5 – Encourage improvement
6 – Use evidence to make decisions
7 – Manage your corporate relationships
Why Choose Global Standards for I.S.O 9001 Certification or quality certification?
Our clients consistently achieve certification because we:
- Tailor solutions to industry-specific needs.
- Engage leadership to ensure top-down commitment.
- Provide hands-on support, not just theoretical guidelines.
- Guarantee 100% success with a structured, phased approach.
Don’t just take our word for it—check our Google reviews and client testimonials on our website.
White Paper - ISO 9001 Quality Management System
NEW METHODOLOGY OF PDCA MODEL
Structure
ISO 9001 now uses Annex SL’s high-level structure (HLS), which establishes a unified framework for all ISO management systems. As a result, this innovative approach provides three key benefits. First, it maintains consistency across standards. Second, it fully aligns different management systems. Third, it standardizes sub-clauses and terminology across all standards for quality certification. Because of this, organizations achieve seamless integration while lowering compliance complexity. Furthermore, this common structure helps businesses implement, maintain, and audit multiple management systems more efficiently than ever before.
PDCA MODULE
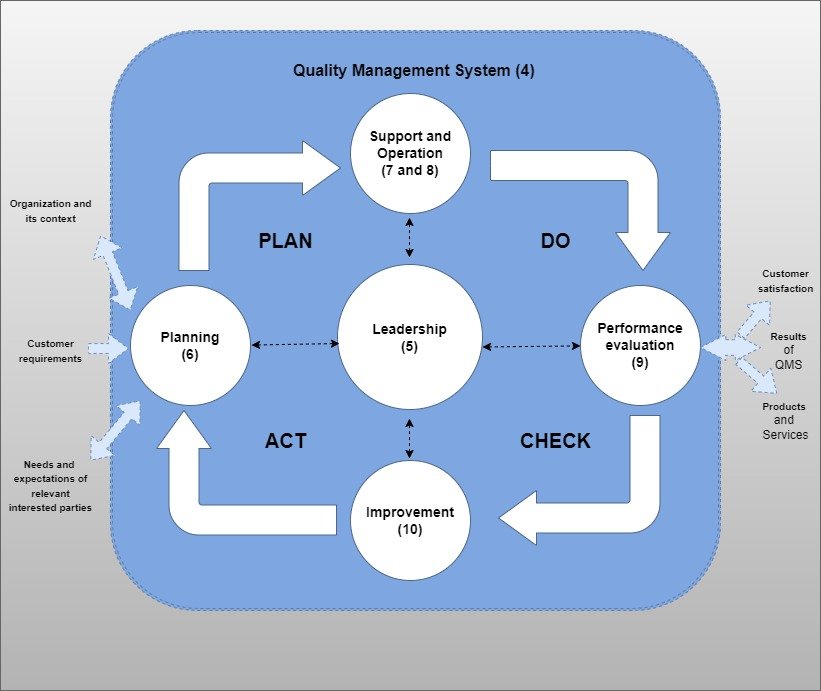
Applicable Clauses for Implementation
- Context of Organization
- Leadership
- Planning
- Support
- Operation
- Perfomance Evaluation
- Improvement
Certification Audit Timeline & Process
Global Standards will lead the organization through a structured ISO 9001 certification process with precision and expertise. First, we conduct a comprehensive Gap Analysis, comparing your current Quality Management System against ISO 9001 requirements to identify improvement areas. Next, we develop a tailored action plan with clear deadlines, ensuring all gaps are addressed before formal audits begin.
During implementation, our team provides hands-on support—helping document processes, train staff, and establish key quality controls. Once your system is ready, we schedule the Stage 1 Audit (Documentation Review), where auditors verify that your policies and procedures meet ISO 9001 standards. After resolving any findings, we proceed to the Stage 2 Audit (On-Site Assessment), evaluating how effectively your QMS operates in practice, including:
- Process effectiveness
- Risk-based thinking
- Customer focus
- Continuous improvement
Throughout the audit, we provide real-time guidance to address non-conformities immediately. Upon successful completion, Global Standards recommends certification to the accreditation body. Following certification, we support you through surveillance audits to maintain compliance and drive ongoing quality enhancements.
By combining rigorous auditing with proactive consulting, we ensure your organization achieves—and sustains—ISO 9001 certification efficiently, demonstrating your commitment to operational excellence and customer satisfaction.
Achieve Certification with Confidence
QMS ISO 9001 is not just a badge it’s a strategic tool for growth, efficiency, and customer satisfaction. Global Standards makes the journey seamless, whether you’re in healthcare, pharmaceuticals, logistics, or any other sector.
Our track record speaks for itself:
- Dr. Ziauddin Hospital achieved full compliance in record time.
- Otsuka Pakistan Ltd boosted QMS efficiency by 70%.
- Pak Shaheen Container Services transformed operations with an 80% improvement.
Ready to elevate your business with ISO 9001 Certification or ISO 9001 Registration? Partner with Global Standards today and experience a results-driven approach to quality excellence.
Contact us now for a free consultation!
The quick contact to Global Standards shall be in benefit to
introduce your organization for true means of ver. 2015 with
effective implementation. Global Standards is passionate to
deliver for the change and integration with similar standards.

For Call or WhatsApp +92-306-2708496
© Global Standards. All rights reserverd for this documented information shared for reading purpose only.
